Good Distribution Practice for Medical Devices GDPMD About GDPMD With the effective date 1 July 2013 of Act 737 and Medical Device Regulations 2012 manufacturers and distributors of. The Good Distribution Practice for Medical Devices GDPMD applies to all companies carrying out activities as stated in the Medical Devices Act 2012 Act 737.
Location map Phone.
. Majid 2019 Good Regulatory. The document which addresses certain quality safety and performance. Provisions in the guideline.
The document which addresses certain quality safety and. When the distribution chain is interrupted by manufacturing steps such as repackaging or relabelling the principles of Good Manufacturing Practice GMP should be applied to these processes. GOOD DISTRIBUTION PRACTICE FOR MEDICAL DEVICES GDPMD GDPMD is a regulatory requirement that affects parties involved in the distribution of medical devices from.
Senior EHS Regulatory Manager 3M Malaysia Sdn Bhd CoCo ee e be a ays a ed ca e ce ssoc a ommittee Member Malaysia Medical Device Association email. Guidelines on Good Distribution Practice GDP Page 1 of 47 GUIDELINES ON GOOD DISTRIBUTION PRACTICE GDP NATIONAL PHARMACEUTICAL CONTROL BUREAU MINISTRY OF HEALTH MALAYSIA Lot 36 Jalan Universiti 46200 Petaling Jaya Selangor Darul Ehsan. Qualification and Validation including 21 CFR Part 11 4.
Validation Parts for Distribution - Practices in different industries. Good distribution practice GDP describes the minimum standards that a wholesale distributor must meet to ensure that the quality and integrity of medicines is maintained throughout the. Manufacturers require ISO13485 Medical Devices Quality Management System certification whereas Distributors and Importers require Good Distribution Practice for Medical Devices.
GDPMD specifies the requirements for a quality management system to be established implemented and maintained by an establishment of medical device importerdistributor. Good Distribution Practices - Medical. Malaysian Good Distribution Practice GDP Guidelines.
830am 530pm 1 full day Delivery Mode. Cyberjaya 15 December 2021 Medical Device Authority Malaysia MDA has organised two days online training on Good Distribution Practice for Medical Devices GDPMD. Building 2 SIRIM Complex 1 Persiaran Dato Menteri.
Lot 36 Jalan Universiti 46200 Petaling Jaya Selangor. Good Regulatory Practice in Malaysia Abdul Latif Hj. Malaysias Medical Device Authority MDA has issued guidance on good distribution practices GDP for medical devices.
Good Distribution Practices GDP Certification for Pharmaceuticals demonstrates your dedication to good distributive practices and quality in every aspect of your service. Good Distribution Practice in Medical Device forum Good Distribution Practice in Medical Device. GOOD DISTRIBUTION PRACTICES FOR MEDICAL DEVICES GDPMD UNDERSTANDING IMPLEMENTING.
Good distribution practice GDP for medical devices training from SGS introducing you to the standard requirements for medical device safety and performance. GDPMD is a stipulated requirement under the Malaysian Medical Act and its accompanying. Abu Seman and Mohd Yazid Abdul Majid May 2019 This chapter should be cited as Seman A.
Incorporated in 1995 Pharmaforte is today a leading local healthcare company that provides value-added services in the marketing sales warehousing. This guideline is applicable to all organisations and individuals. Elucidate the requirements for an appropriate management and control of these.
Malaysia Good Distribution Practice for Medical Device GDPMD Date. SIRIM STS Sdn Bhd. MINISTRY OF HEALTH MALAYSIA GOOD DISTRIBUTION PRACTICE FOR MEDICAL DEVICES GDPMD Appendix 4 Schedule 3 Medical Device Regulation 2012 Seminar Updates on.
The companies involved in. Annex 9 Model guidance for the storage and transport of time- and temperaturesensitive. Malaysias Medical Device Authority has issued guidance on good distribution practices for medical devices.
The Good Distribution Practice for Medical Devices GDPMD is developed to.
Sustainability Free Full Text Role Of Social And Technological Challenges In Achieving A Sustainable Competitive Advantage And Sustainable Business Performance Html
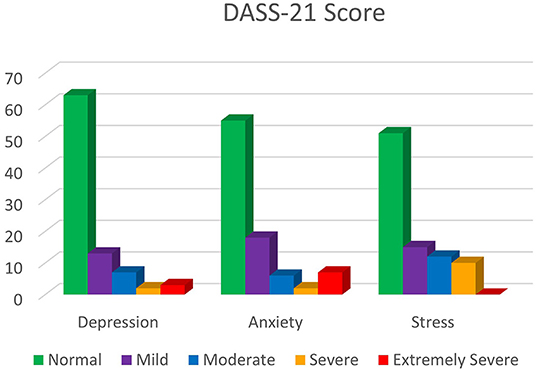
Frontiers Promoting Mental Health During The Covid 19 Pandemic A Hybrid Innovative Approach In Malaysia Public Health

An Overview Of Palm Oil Biomass For Power Generation Sector Decarbonization In Malaysia Progress Challenges And Prospects Zamri Wires Energy And Environment Wiley Online Library

Pharmaboardroom Marketing Manufacturing Packaging Labeling Advertising Malaysia
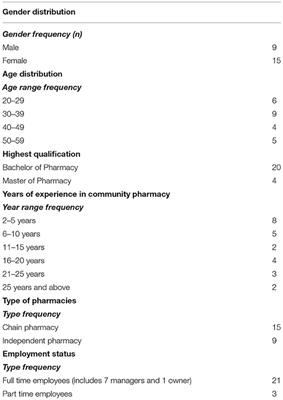
A Qualitative Study Exploring Community Pharmacists Experiences And Views About Weight Management Interventions And Services In Klang Valley Malaysia Public Health Frontiers
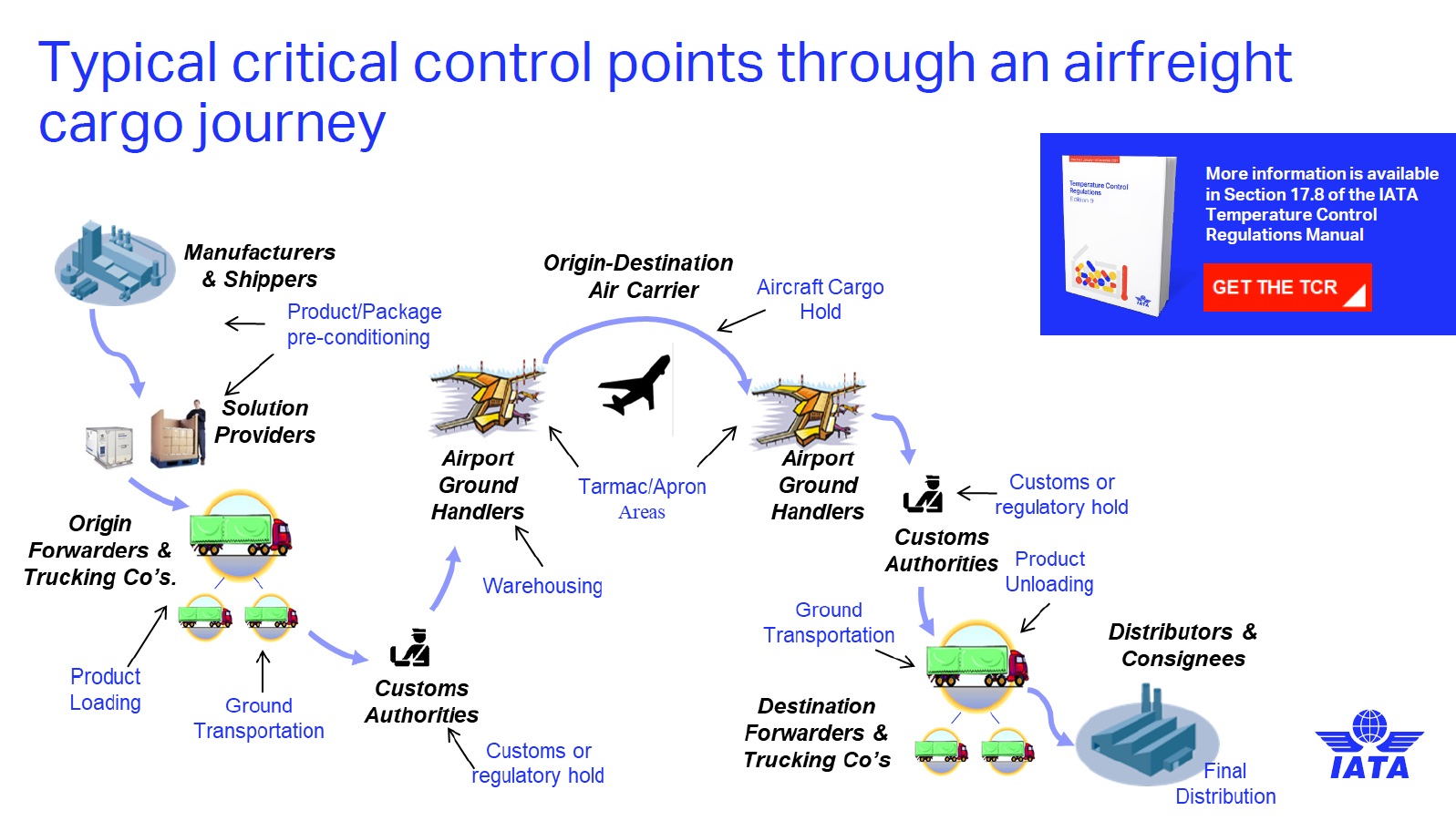
Iata What Does The Healthcare Industry Look For When Choosing A Temperature Controlled Transportation Partner

Pharmaboardroom Marketing Manufacturing Packaging Labeling Advertising Malaysia
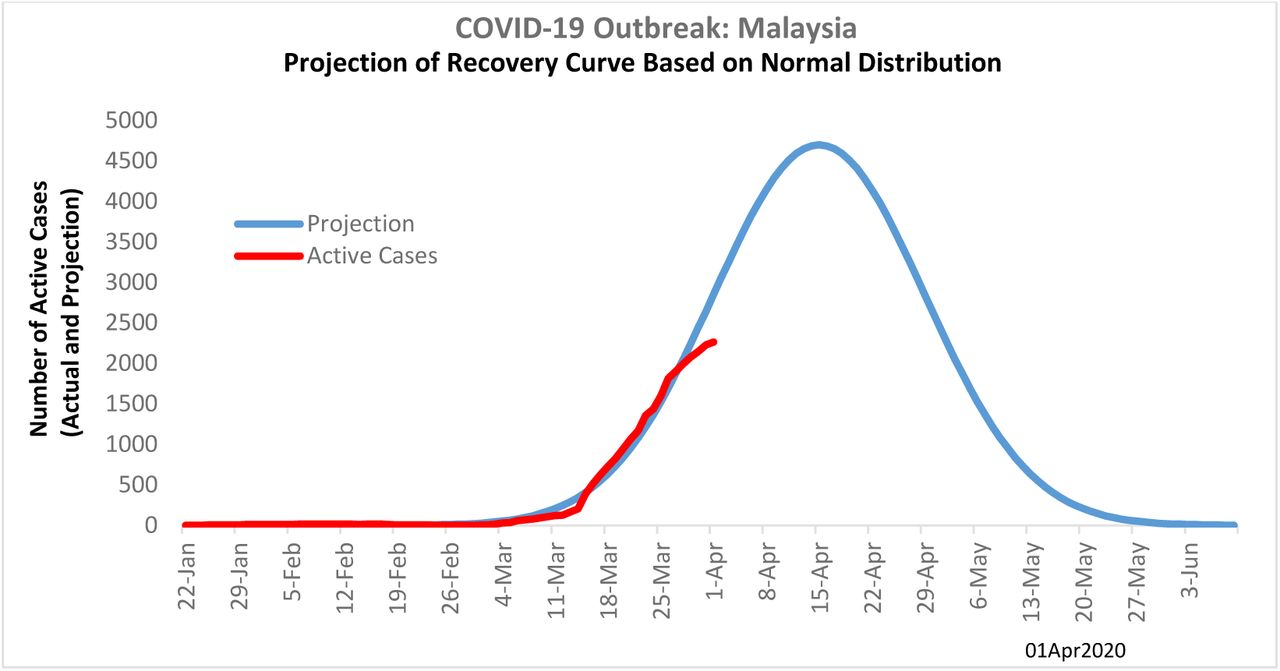
Covid 19 Epidemic In Malaysia Impact Of Lockdown On Infection Dynamics Medrxiv
Towards Sustainable Transport Policy Framework A Rail Based Transit System In Klang Valley Malaysia Plos One
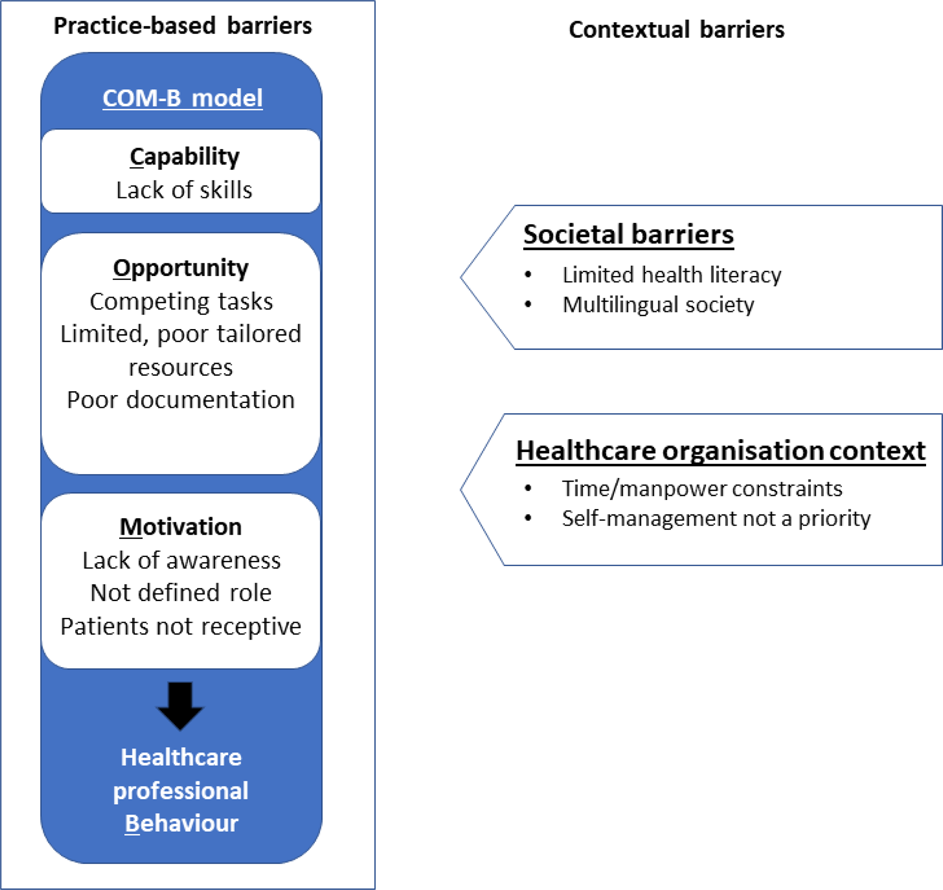
Barriers To Implementing Asthma Self Management In Malaysian Primary Care Qualitative Study Exploring The Perspectives Of Healthcare Professionals Npj Primary Care Respiratory Medicine

Malaysia S Political Polarization Race Religion And Reform Political Polarization In South And Southeast Asia Old Divisions New Dangers Carnegie Endowment For International Peace

Biocare Group M Sdn Bhd Health And Nutritioun
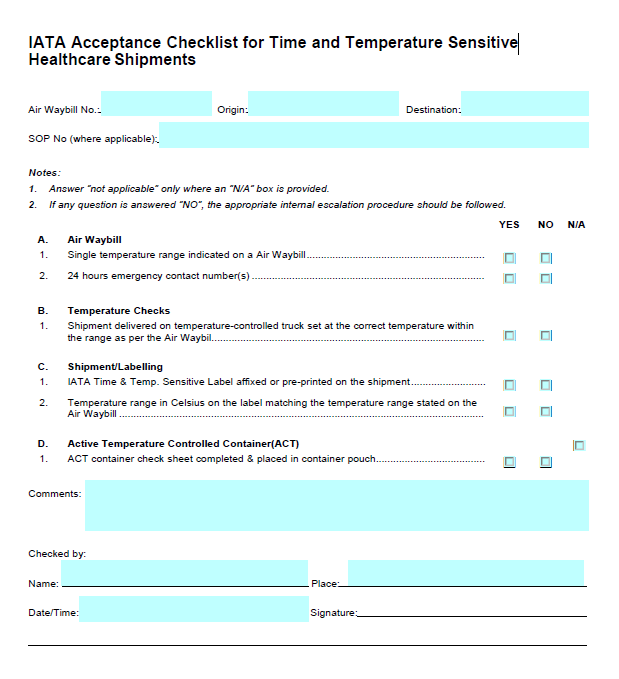
Iata What Does The Healthcare Industry Look For When Choosing A Temperature Controlled Transportation Partner

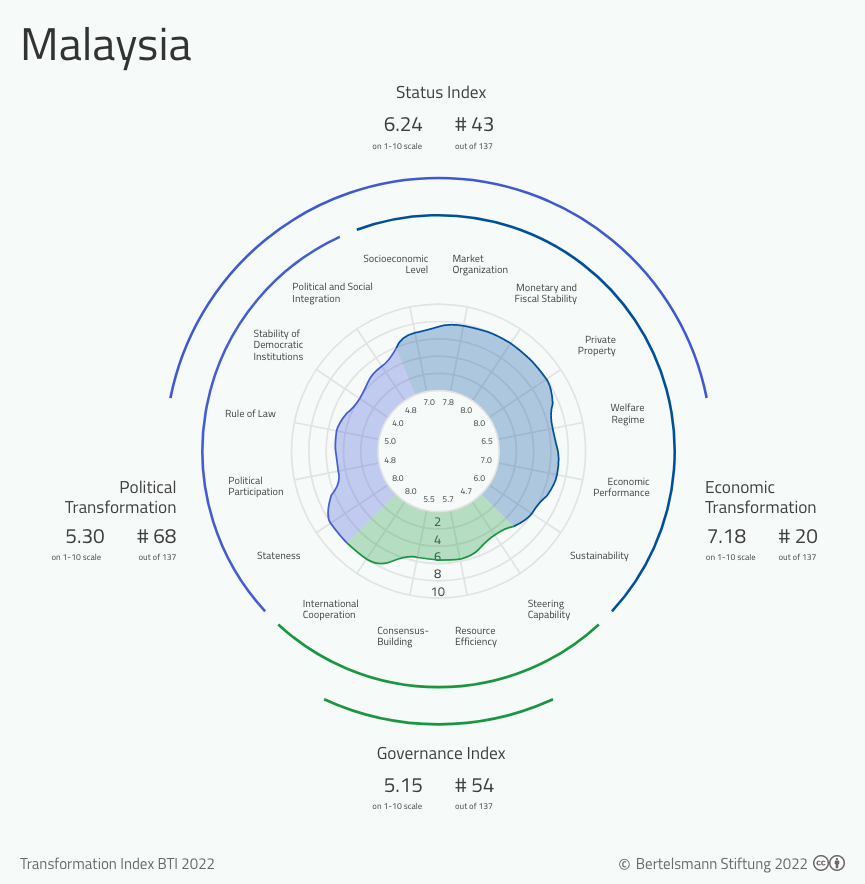
Bti 2022 Malaysia Country Report Bti 2022

Supplementary Training Modules On Good Manufacturing Practices Health Technology Good Manufacturing Practice Energy Management

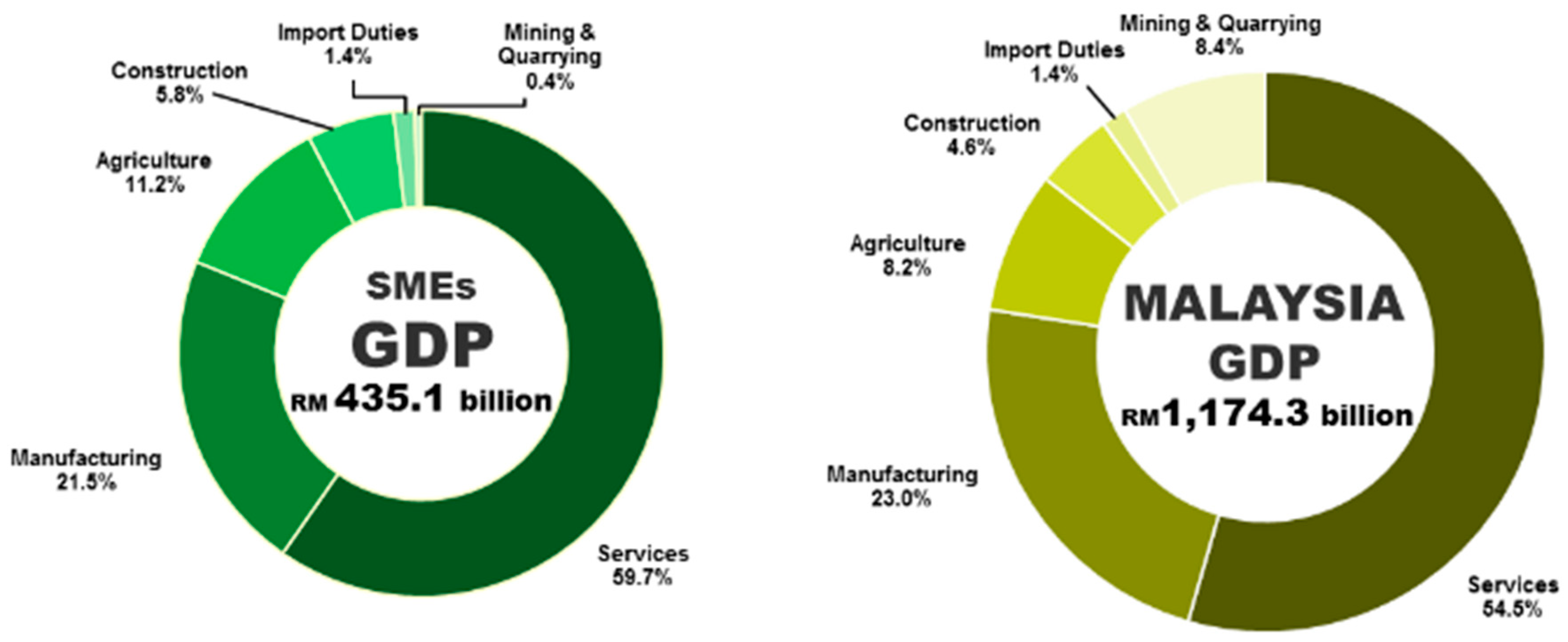
Pdf The Role Of Financial Behaviour Financial Literacy And Financial Stress In Explaining The Financial Well Being Of B40 Group In Malaysia


